Cycloheptapeptides with promise as Anti-TB drug leads
Introduction
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, remains one of the most significant infectious diseases worldwide, with a pressing need for new therapeutic agents to combat both drug-sensitive and drug-resistant strains. This urgency has led to the exploration of cycloheptapeptides, a class of compounds exhibiting potent antimycobacterial activities, with promising candidates like ecumycin and rufomycin showing effectiveness against various TB strains.
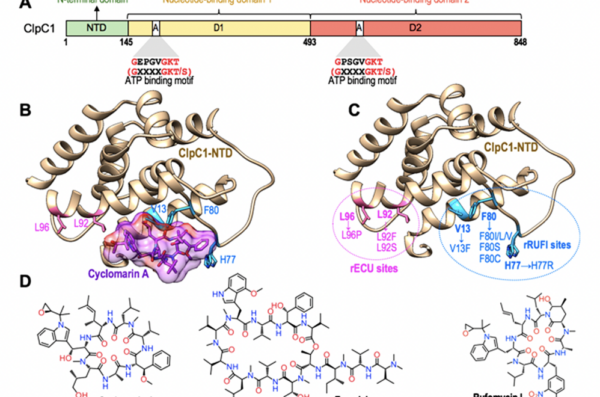
Cycloheptapeptides, such as ecumycin and rufomycin, represent a new frontier in anti-TB drug discovery. These compounds exert their bactericidal effects by targeting ClpC1, an ATPase associated with diverse cellular activities (AAA) protein that plays a pivotal role in the proteolysis of proteins within the bacterial cell. By inhibiting ClpC1, these cyclic peptides disrupt the protein degradation pathway essential for the survival and virulence of Mycobacterium tuberculosis, thereby offering a novel mechanism of action compared to traditional TB therapies.
The specificity and potency of cycloheptapeptides against TB, including multi-drug resistant (MDR) and extensively drug-resistant (XDR) strains, make them particularly attractive as drug leads. Their unique mode of action also reduces the likelihood of cross-resistance with existing TB drugs, which is a critical advantage in the current landscape where resistance is a growing concern.
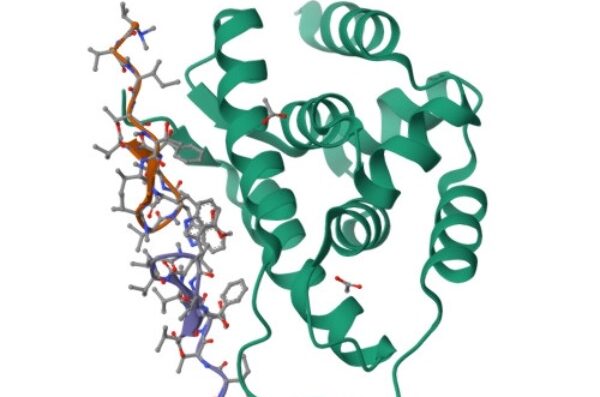
The figures below illustrate the structures of the end terminal domain (NTD) of the target ClpC1 bound to the most well characterized cyclopeptides (ecumicin, rufomycin). The structures are available on Protein Data Bank as entries 6PBS, 6CN8.